Introduction: EMA Highlights Ozempic Wegovy Eye Risk
The Ozempic Wegovy eye risk has now been validated by the European Medicines Agency (EMA), marking a major update for users of these blockbuster semaglutide drugs. While still classified as a very rare side effect, the EMA’s Pharmacovigilance Risk Assessment Committee (PRAC) advises adding non-arteritic anterior ischemic optic neuropathy (NAION) to product warnings. This is the first time a regulator has officially recognized this serious condition.
What Is the Ozempic Wegovy Eye Risk? Understanding NAION
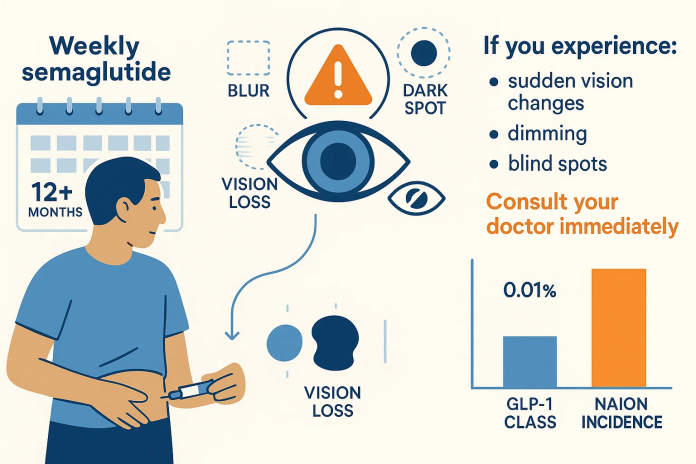
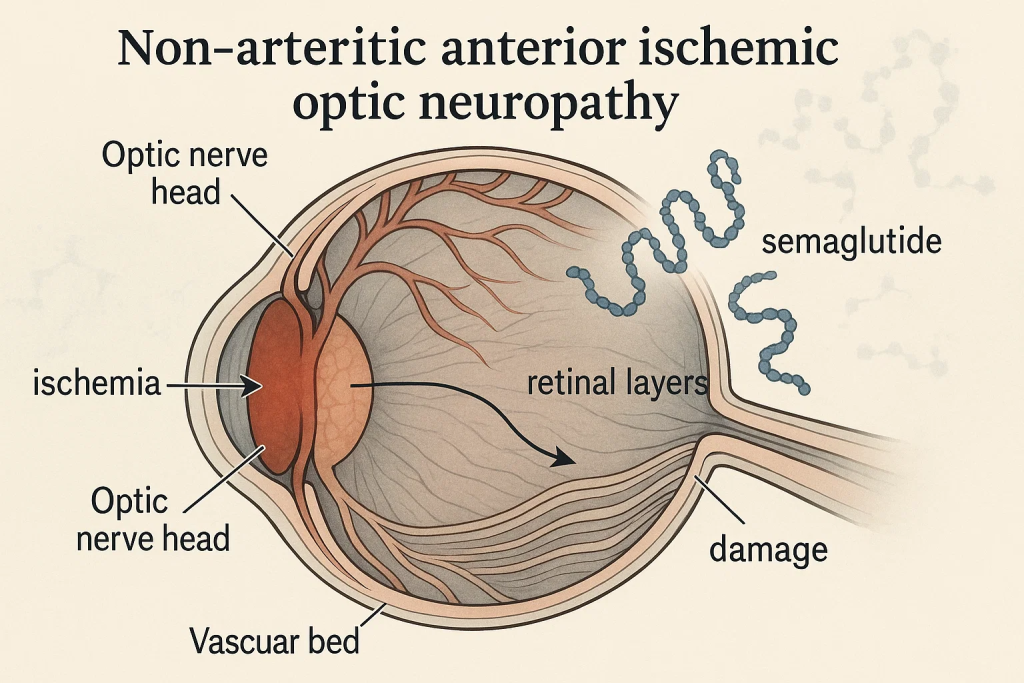
NAION occurs due to reduced blood flow to the optic nerve, resulting in sudden, painless vision loss—often in a single eye. It’s the second most common cause of optic nerve blindness after glaucoma.
- Incidence rate: EMA reports NAION may affect up to 1 in 10,000 semaglutide users, including those taking Ozempic, Wegovy, or Rybelsus, after at least one year of treatment
- Risk elevation: Large epidemiological studies demonstrate approximately a two-fold increase in NAION risk among semaglutide-treated type 2 diabetes patients compared to those on other diabetes medications
EMA’s Regulatory Response to Ozempic Wegovy Eye Risk
After a comprehensive review—covering clinical trials, post-marketing surveillance, and medical literature—the EMA has:
- Classified NAION as a “very rare” side effect of semaglutide-based treatments
- Recommended updating all product labels to include “Ozempic Wegovy eye risk” warnings and advising treatment discontinuation if sudden vision loss occurs.
- Urged healthcare providers to advise patients to monitor vision changes closely and seek immediate medical attention
Industry Response and Expert Perspective
- Novo Nordisk confirmed it would add the NAION warning to labels, while reiterating the benefit-risk profile of semaglutide remains favorable
- Emily Field from Barclays highlighted that clinicians have been aware of the risk, stating it is “unlikely to significantly alter prescribing patterns”
- U.S. authorities, including the FDA, have not issued a formal response or launched an investigation—though they may review the EMA’s findings.
Clinical Evidence Behind the Ozempic Wegovy Eye Risk
Key data supporting the Ozempic Wegovy eye risk includes:
- A March study involving 350,000 diabetes patients showed NAION risk more than doubled after two years of semaglutide use compared to those on other drug classes
- Additional JAMA Ophthalmology research and a Danish/Norwegian preprint confirmed similar risk patterns—though the absolute risk remains low (~0.01%, or 1 in 10,000)
- Prior reports linked semaglutide to ocular issues after prolonged usage, supporting EMA’s decision
What Should Patients and Clinicians Do?
For Patients:
- Stay informed about Ozempic Wegovy eye risk and recognize early signs of vision loss.
- Immediately consult your doctor if you experience blurred vision, dark spots, or impaired eyesight.
- Do not stop medication without professional guidance.
- Report any vision changes to both your healthcare provider and the drug manufacturer.
For Clinicians:
- Discuss the risk during consultations with patients on semaglutide.
- Conduct baseline eye exams, particularly for older patients or those with vascular risk factors.
- Monitor vision and consider early referral to ophthalmology if symptoms arise.
- Report any adverse events in your region’s pharmacovigilance database.
Broader Implications and Future Outlook
Despite the Ozempic Wegovy eye risk, semaglutide therapies continue to dominate the obesity and diabetes market—expected to reach $150 billion globally. However:
- Label updates will raise awareness for both clinicians and patients in the EU.
- Indoors to FDA safety assessments could follow, shaping U.S. prescribing guidance.
- Further research is needed to determine whether NAION risk extends to other GLP‑1 analogs like Mounjaro and their influence on retinal health.
Conclusion: Balancing Benefits With Rare Risks
The EMA’s official recognition of Ozempic Wegovy eye risk underscores the importance of vigilance—but doesn’t diminish the drugs’ therapeutic value. With early detection and prompt action, patients on semaglutide can continue to benefit from its proven glucose-lowering and weight-loss effects while minimizing the chance of rare ocular harm.
Sources:
- Reuters – Ozempic, Wegovy may cause rare eye condition, EMA says (June 6, 2025)
- Reuters – Health Rounds: Ozempic again linked to sight-threatening eye condition in diabetes study (March 28, 2025)
- Huffington Post España – La EMA alerta de un tipo de ceguera como efecto secundario muy raro de Ozempic (June 6, 2025)
- EMA – European Medicines Agency: Pharmacovigilance Risk Assessment Committee (PRAC) Monthly Highlights
- JAMA Ophthalmology – Studies on semaglutide and ocular adverse events (2024–2025)
- Novo Nordisk – Investor and Safety Updates (2025)