Introduction: Game-Changer for dMMR Stage 3 Colon Cancer
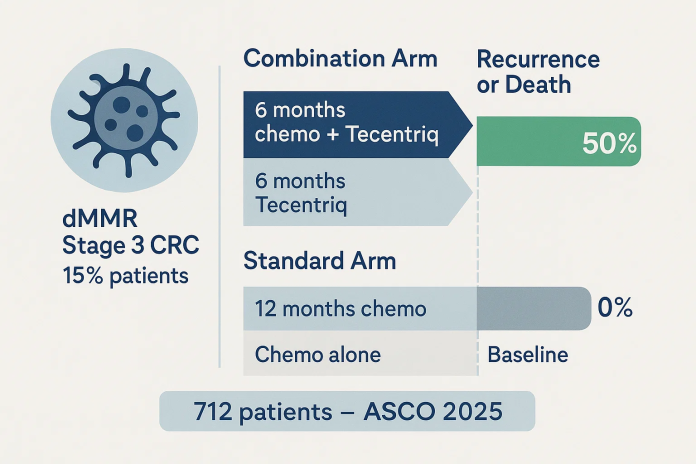
The Roche Tecentriq colon cancer study presented at the ASCO meeting marks a breakthrough in managing stage 3 colorectal cancer with deficient DNA mismatch repair (dMMR). When Tecentriq is added to chemotherapy post-surgery, it cut recurrence and death risk by a notable 50%, a milestone that could transform treatment guidelines.
Trial Design: Rigorous Testing in dMMR Patients
The multicenter Phase 3 trial involved 712 patients with surgically treated stage 3 colon cancer featuring lymph node involvement and dMMR tumors—15% of colon cancer cases. Participants were randomized:
- Group A (Combination): 6 months of chemotherapy + Tecentriq, followed by 6 months of Tecentriq monotherapy
- Group B (Standard): 12 months of chemotherapy alone
Significant Results: 50% Risk Reduction
- Recurrence/death was halved in the Tecentriq group relative to chemotherapy alone
- Benefits were consistent across age and risk factors
“It’s extremely rewarding to offer our patients a new treatment regimen that can reduce the risk of recurrence and improve their chances of survival,” said Dr Frank Sinicrope of Mayo Clinic
These results suggest adjuvant immunotherapy can significantly enhance outcomes in high-risk dMMR colon cancer.
Why the Roche Tecentriq Colon Cancer Trial Matters
Patients with dMMR tumors typically show poor response to chemotherapy alone. This trial represents a leap forward by combining immunotherapy with traditional treatments in an adjuvant setting.
Tecentriq’s mechanism—activating immune responses against residual cancer cells—acts on microscopic disease that standard therapy may not completely eliminate, improving long-term prognosis.
Broader Clinical Impact
- Establishes a new standard of care: Likely to influence NCCN and ASCO treatment guidelines
- Benefit across hot subgroups: Includes elderly and high-risk patients
- Precision medicine milestone: Validates targeting therapies based on tumor genetics
Future Steps: Regulatory & Long-Term Follow-Up
- Roche plans to submit these Phase 3 findings to regulators (FDA, EMA) for label updates
- Additional follow-up data—including overall survival and long-term safety—will be crucial
- Potential expansion of Tecentriq to other dMMR cancers like endometrial and gastric malignancies
Conclusion: A Milestone in Colon Cancer Care
The Roche Tecentriq colon cancer study sets a new bar—halving the risk of recurrence and death in stage 3 dMMR patients. As immunotherapy integrates into standard protocols, this could redefine care for a high-need patient group. With regulatory submissions underway, the oncology community eyes long-term impacts and future applications.