Introduction: U.S. COVID Vaccine Recommendations Spark Confusion
The newly announced alterations in U.S. COVID vaccine recommendations, first signaled by Health Secretary Robert F. Kennedy Jr., have sown confusion among clinicians, insurers, and the public. Diverging messaging on vaccination for healthy children and pregnant women—announced via social media and then modified on the CDC’s website—has left healthcare stakeholders uncertain about what constitutes current policy and who will be covered under insurance plans.
Background: Timeline and Key Actions
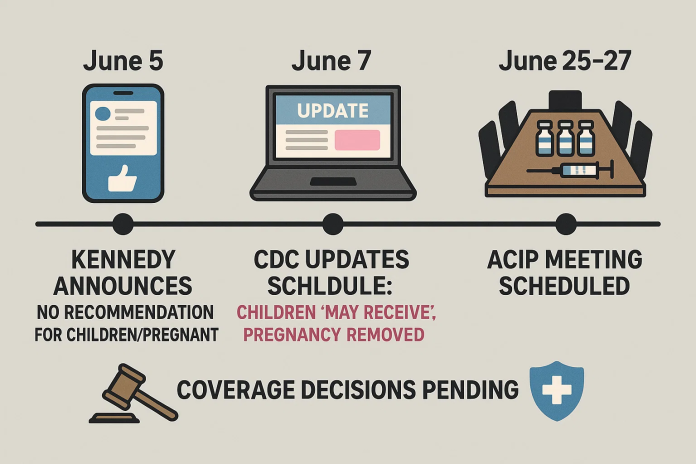
- On June 5, 2025, Secretary Kennedy announced on social media that the federal government was no longer recommending COVID‑19 vaccines for healthy children and pregnant women. This bypassed the usual route through the CDC’s Advisory Committee on Immunization Practices (ACIP).
- Days later, the CDC updated its immunization schedule stating vaccination may be given to healthy children after clinical discussion but removed support for pregnant women.
- This marked departure from protocol—ACIP normally conducts public deliberations and votes that inform CDC policy, which in turn influences Affordable Care Act (ACA) insurer coverage.
Why the Changes Trigger Confusion
For Doctors and Public Health Officials
Many clinicians are unsure whether to continue recommending shots. As one ACIP member told Reuters, “It’s very unclear whose decision is final.” The disruption to established procedure has also prompted the resignation of Dr. Lakshmi Panagiotakopoulos, a CDC vaccine adviser, who said she can no longer “help the most vulnerable” under this uncertainty.
For Insurers
Insurers rely on the CDC schedule to determine coverage. Without formal ACIP guidance, they face liability concerns and are delaying coverage decisions ahead of ACIP’s upcoming June 25–27 meeting.
For Patients and Parents
Vaccination access for children and pregnant women is murky. Organizations like the American College of Obstetricians and Gynecologists have warned that the gap in recommendations could create barriers when evidence supports vaccination during pregnancy.

Legal & Policy Implications
HHS asserts its approach is “within legal authority” and emphasizes that COVID vaccination remains a clinic-based, patient-provider decision. Still, deviating from ACIP procedure has legal implications, since ACA mandates insurer coverage based on ACIP-endorsed vaccines. Experts caution that insurers postponing coverage until after ACIP could limit access to boosters this fall.
ACIP Meeting on the Horizon
ACIP is scheduled to meet June 25–27, and is expected to revisit recommendations for the fall-winter COVID-19 booster rollout. The panel is reportedly leaning toward guidance for high-risk populations only, including older adults and immunocompromised individuals. CDC will likely update its official schedule post-meeting.
Impacts & Reactions
- Public Trust: The shift has been widely criticized as eroding public confidence in health agencies.
- Professional Voices: Organizations like the Infectious Diseases Society of America and ACOG warned the changes could complicate care delivery to vulnerable groups.
- Practical Barriers: Parents of children in vaccine trials have decried the policy as unfair, urging that clinical progress and data be respected.
Conclusion: Navigating the Uncertain Landscape
The unfolding situation around U.S. COVID vaccine recommendations reflects a rare divergence from standard policy-making norms. Though intended to promote clinical discretion, the moves have left providers, insurers, and the public seeking clarity. As ACIP reconvenes, transparent processes and expert consensus will be key to restoring trust and ensuring access for those in need.
Sources:
- Reuters – “US COVID vaccine recommendations sow confusion among doctors, insurers”
- The Washington Post – “Top CDC COVID vaccine adviser quits after RFK Jr. ended recommendations”
- San Francisco Chronicle – “Confused over the conflicting COVID vaccine guidance? Here’s what to know”
- CDC – Immunization Schedules (Updated June 2025)
- ACOG – American College of Obstetricians and Gynecologists: COVID-19 Vaccine and Pregnancy Position Statement
- Health Affairs – “COVID-19 Vaccine Coverage and the ACA: How Insurers Use ACIP Guidelines”